Cacl2 Intermolecular Forces. Solid calcium chloride is deliquescent, meaning it can absorb enough moisture to convert to liquid brine. And this occurs when hydrogen is bound to a strongly electronegative element such as oxygen, or nitrogen, or fluorine… and the simple.
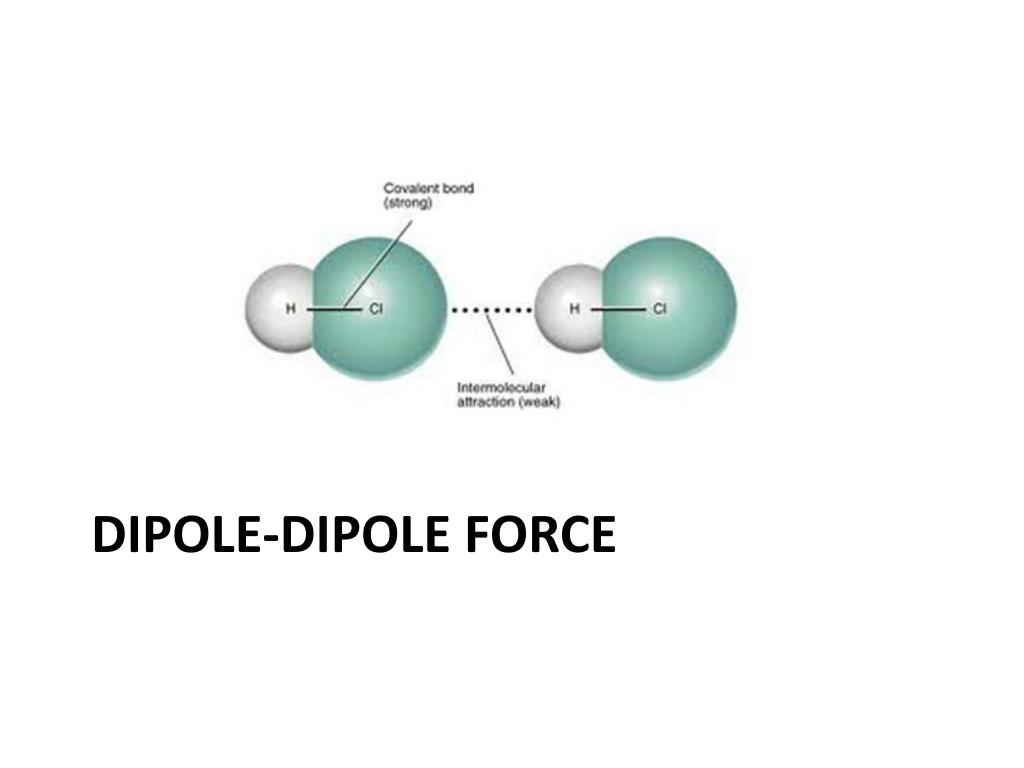
What intermolecular forces occur between the following? Technically there are no intermolecular forces in cacl2 because as an ionic compound, it consists of a lattice of positive and negative ions held together by strong coulombic forces called ionic bonds. What is the strongest intermolecular force for cacl2?!
D) Increasing The Pressure On A Solid Usually Causes It To Become A Liquid.
Likewise, what types of intermolecular forces exist in nh3? What is cacl2 intermolecular forces. Specific heat capacity enthalpy of vaporization 8.
Two Quantum Mechanical Approximation Formulas Due To Slater And Kirkwood Are Modified Empirically Such As To Relate The Empirical Intermolecular Force Constants Of A Two‐Parameter Potential To The Average Polarizabilities And The Number Of Valence Electrons Of The Interacting Molecules.
Which type of intermolecular forces exist between nacl and h2o? In nh3 there will definitely be hydrogen bonding as you can see from the formula and london forces (also known as dispresion forces) in cacl2 there will be london forces along with dipole dipole. This association leads to a decrease in the vapor pressure and an increase in the boiling point as more energy is required to vaporize the molecule.
Other Examples Include Quartz, Sio 2, Silicon Carbide, Sic, And Boron Nitride, Bn.
The specific heats of ice, water, and steam are 2.09 j/g−k, 4.18 j/g−k, and 1.84 j/g−k, respectively. Solution for illustrate the intermolecular forces of: Technically there are no intermolecular forces in cacl2 because as an ionic compound, it consists of a lattice of positive and negative ions held together by strong coulombic forces called ionic bonds.
What Is The Intermolecular Forces Of Cacl2?
Ccl4 is a nonpolar molecule. When dissolved in water, solid calcium chloride releases heat in an exothermic reaction. And this occurs when hydrogen is bound to a strongly electronegative element such as oxygen, or nitrogen, or fluorine… and the simple.
Intermolecular Forces (Imf) Are The Forces Which Cause Real Gases To Deviate From Ideal Gas Behavior.
What is the strongest intermolecular force for cacl2?! Ammonia is a polar molecule (1.42 d), and so it exhibits all three of the van der waals forces: What kind of force is cacl2?